Imaging for better decisions
From study design to final reporting, Bioxydyn delivers repeatable MRI biomarkers revealing tissue function, physiology and change. Our operational excellence and metrology help you compare results across sites, scanners and timepoints.
Statistics
We're Bioxydyn - delivering quantitative MR biomarkers for pharmaceutical, biotech and academic studies. We combine scientifically leading methods with robust multi-centre study management and transparent analysis, turning complex images into repeatable measurements that support confident decisions.
Founded in 2009, Bioxydyn has been delivering commercial multi-centre clinical trials since.
Sites trained across North America, Europe, Asia, Australia, South America and Africa.
VoxelFlow supports your clinical trial end-to-end, delivering reliable, repeatable and auditable outputs. It is compliant with FDA 21 CFR Part 11
Read about how Bioxydyn can support your Respiratory study
Read about how Bioxydyn can support your Oncology study
Read about how Bioxydyn can support your Inflammation study
Read about how Bioxydyn can support your Neuroimaging study
Read about how Bioxydyn can support your Liver imaging study
Read about how Bioxydyn can support your MR Spectroscopy study
Learn about Publications
Learn about Software Development Services
Learn about Trial design
Learn about VoxelFlow
Learn about Imaging Data Management
Learn about Imaging Site Support
Learn about MR Protocol development & harmonisation
Learn about Quality & Compliance
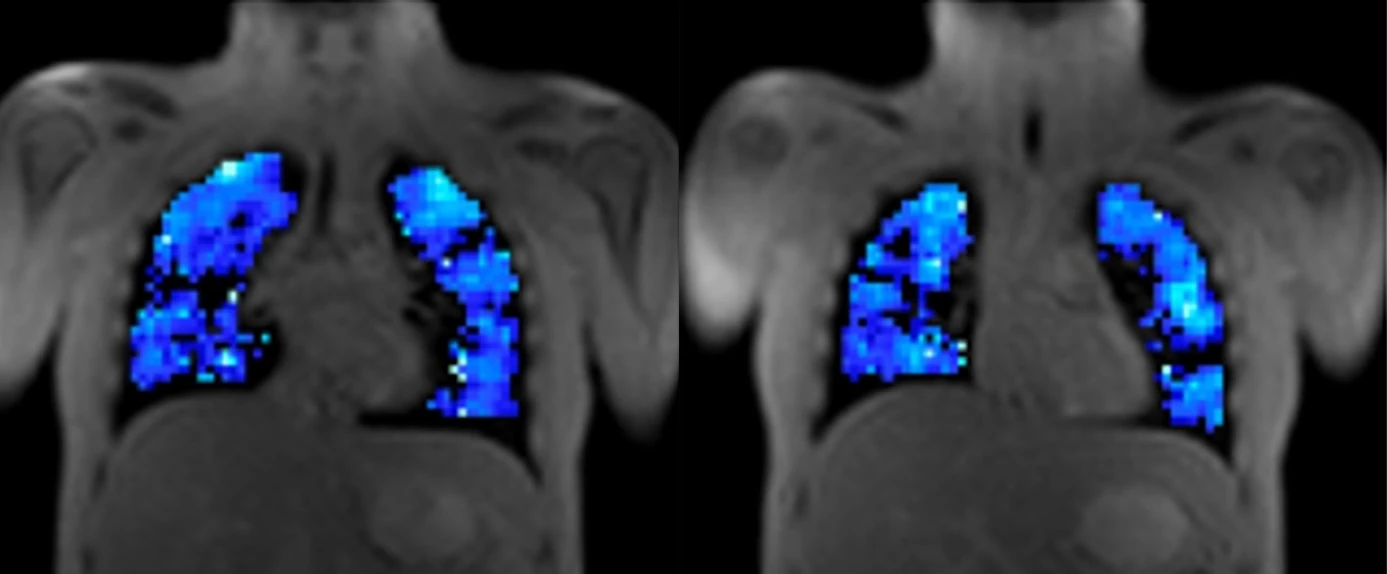
Respiratory
Quantitative MRI and ventilation imaging to support respiratory drug development.
- Oxygen-enhanced MRI (OE-MRI) ventilation biomarkers
- DCE-MRI perfusion and microvascular measures
- Motion-based ventilation imaging without gases
- Support for hyperpolarised 129Xe studies
- Validated methods across vendors and sites
Oncology
Assess tumour hypoxia, perfusion, and therapy response with quantitative imaging.
- Oxygen-enhanced MRI (OE-MRI) for tumour hypoxia
- DCE-MRI biomarkers of angiogenesis and perfusion
- Diffusion MRI (ADC) for early response detection
- Cross-site protocol standardisation and QA
- Auditable analysis and reporting with VoxelFlow
Inflammation
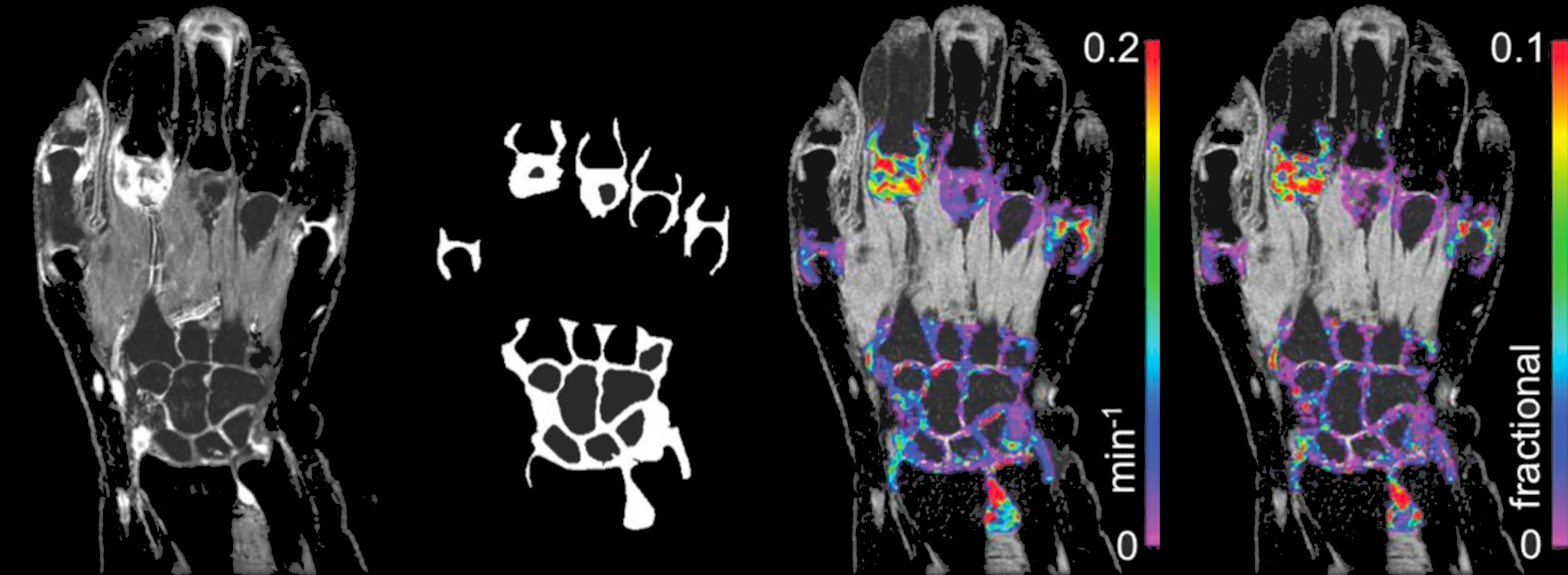
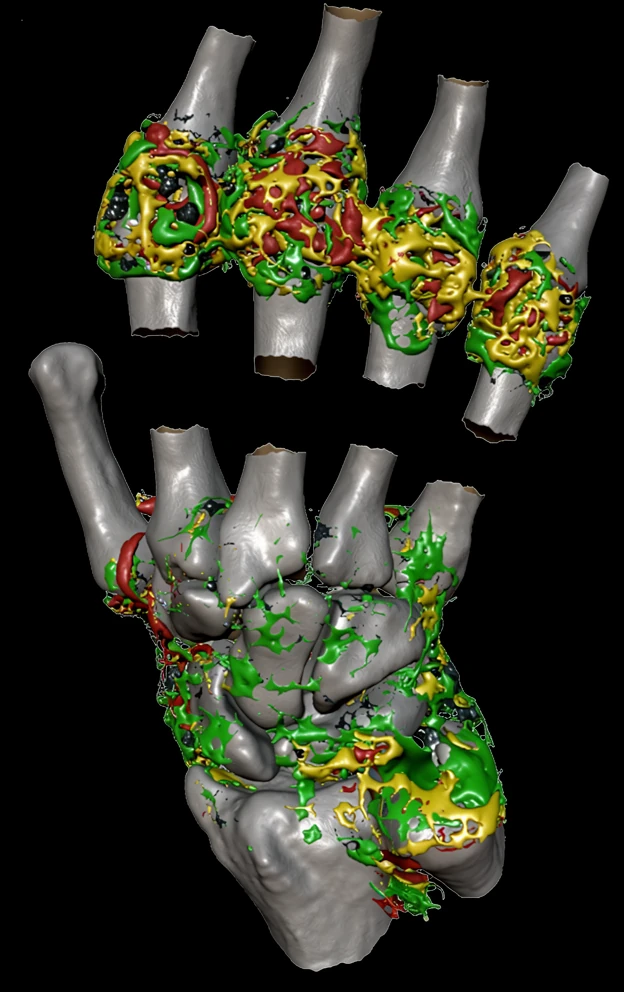
Inflammation biomarkers for arthritis, autoimmune disease, and organ injury.
- DCE-MRI Ktrans biomarkers of microvascular function
- Joint imaging in rheumatoid arthritis and osteoarthritis
- Renal imaging biomarkers including ASL, T1, T2, ADC
- Hypoxia and pH imaging methods (BOLD, OE-MRI, CEST)
- Sensitive, repeatable measurements with small cohorts
Neuroimaging
Brain MRI biomarkers for neurodegeneration, neuro-oncology, and CNS trials.
- Atrophy and diffusion metrics for neurodegeneration
- DCE-MRI for subtle blood-brain barrier dysfunction
- Arterial spin labelling (ASL) perfusion methods
- MR spectroscopy and relaxometry
- AI stratification with Queen Square Analytics
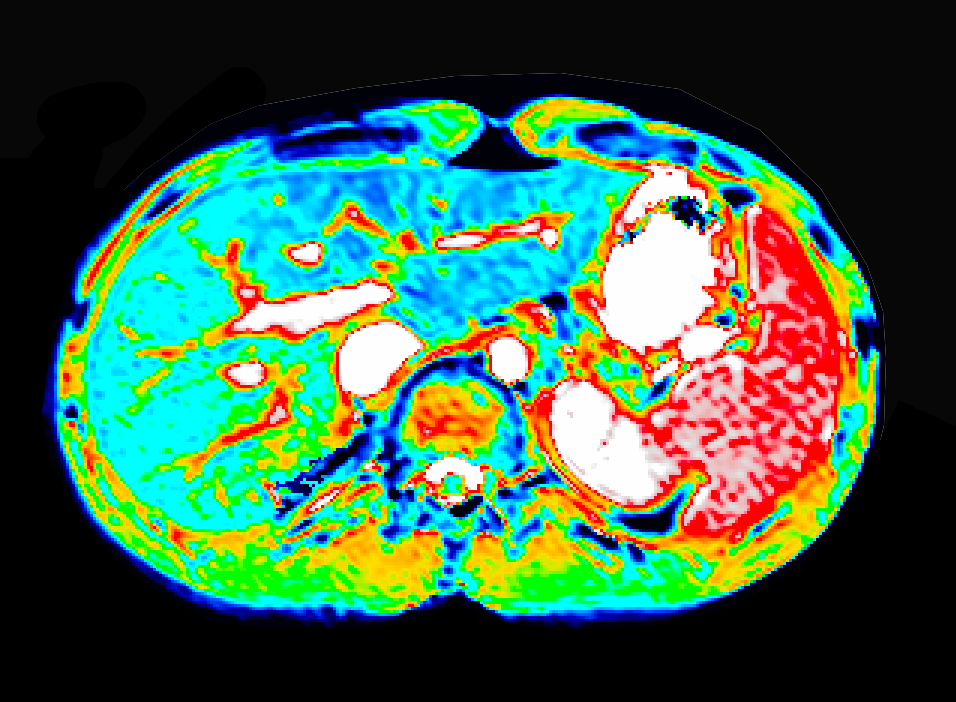
Liver imaging
Non-invasive liver function assessment and fibrosis mapping with MRI.
- Gadoxetate DCE-MRI for uptake and efflux kinetics
- Relaxometry (T1, T2, T2*) for tissue status
- TRISTAN project leadership and validation
- FDA biomarker qualification program acceptance
- Study design, QC, analysis, and reporting
MR Spectroscopy
Metabolic profiling with MR spectroscopy to understand tissue chemistry.
Discover more
Software Development Services
Custom software solutions to streamline imaging workflows and analytics.
Discover more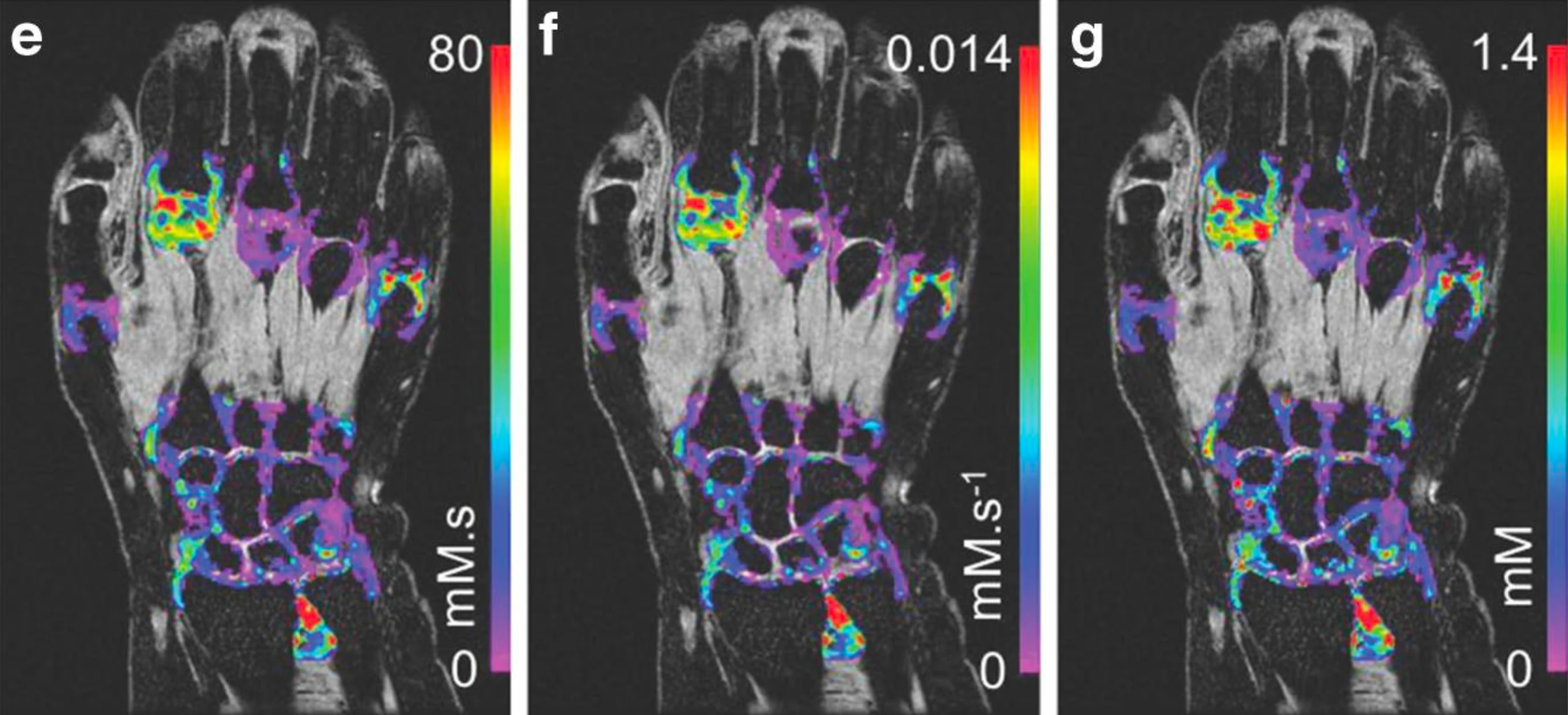
Trial design
Trial design guidance and imaging strategies aligned with clinical endpoints.
Discover more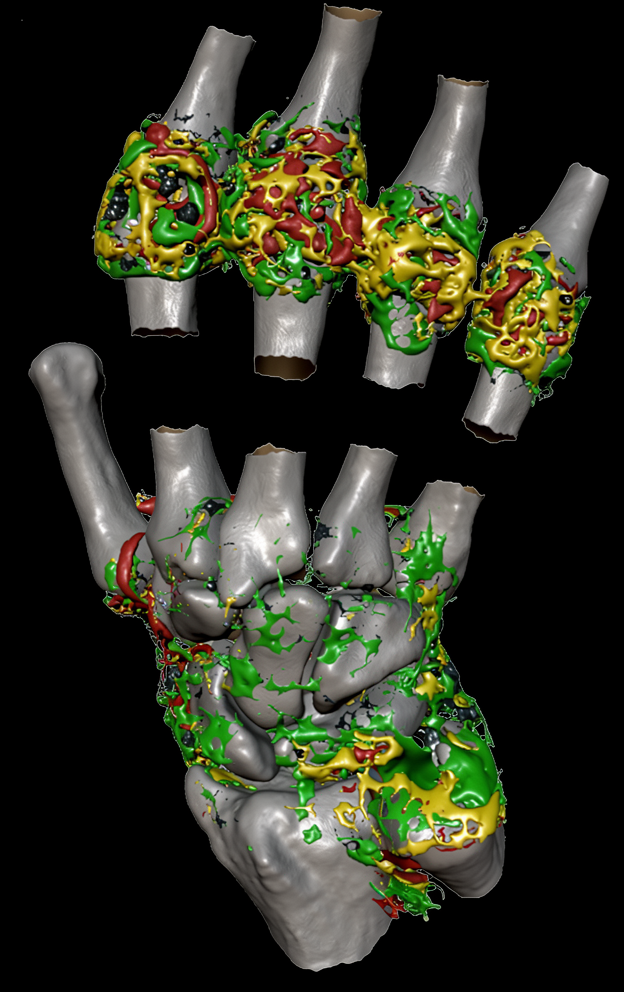


Imaging Site Support
Site training, qualification, and support to reduce imaging variability.
Discover more
MR Protocol development & harmonisation
MR protocol development, harmonisation, and cross-site consistency checks.
Discover more
Quality & Compliance
Quality frameworks and compliance support across imaging programs.
Discover more